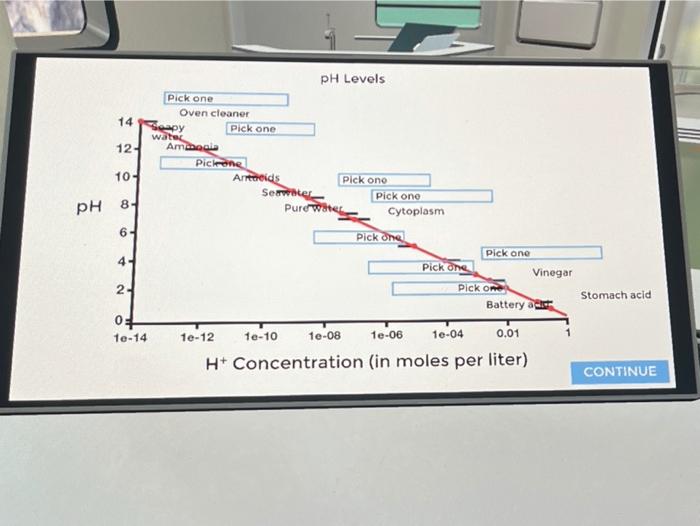
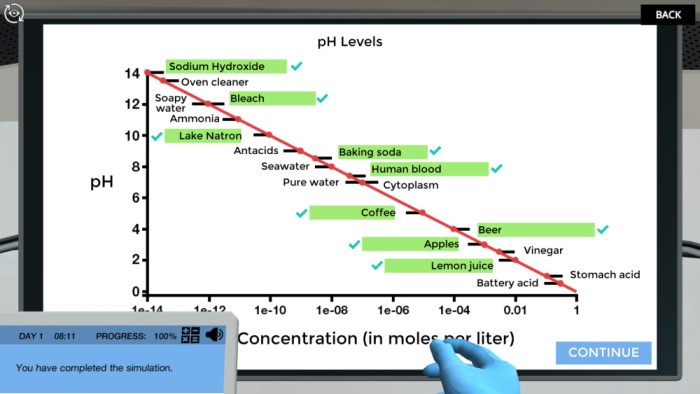
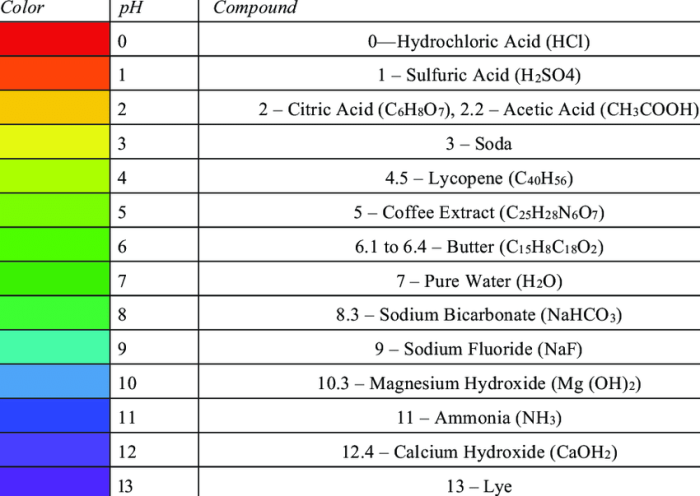
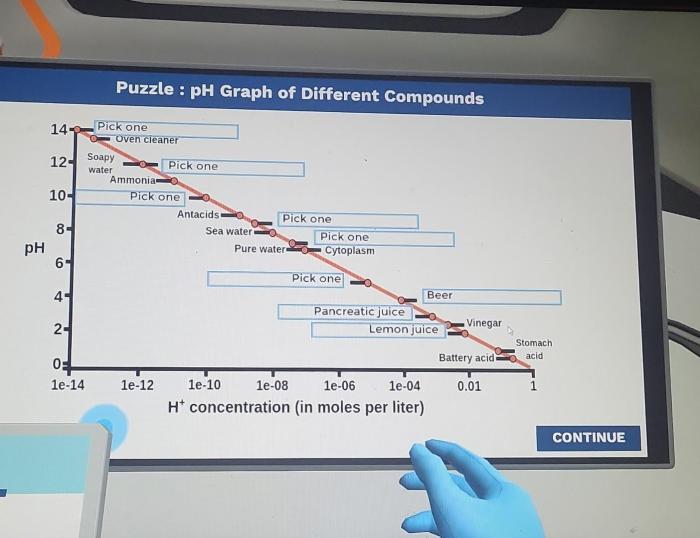
The puzzle pH graph of different compounds offers a fascinating glimpse into the complex world of phase behavior, where the interplay of temperature, pressure, and composition dictates the physical state of matter. This article delves into the intricacies of phase diagrams, exploring their construction, interpretation, and practical applications across scientific disciplines.
Phase diagrams serve as indispensable tools for understanding the phase transitions of various compounds, providing insights into their thermodynamic properties and guiding decision-making processes in diverse fields.
Phase Diagrams: Understanding the Behavior of Different Compounds
Phase diagrams are graphical representations that depict the equilibrium phases of a substance under varying conditions of temperature and pressure. They provide valuable insights into the physical and chemical behavior of materials, making them essential tools in various scientific and engineering disciplines.
Phase diagrams are constructed by plotting the boundaries between different phases, such as solid, liquid, and gas. These boundaries represent the conditions under which the phases coexist in equilibrium. By analyzing phase diagrams, scientists and engineers can determine the phase transitions that occur in a substance, as well as the temperature and pressure ranges at which these transitions take place.
Phase Diagrams for Different Compounds
The phase diagrams of different compounds exhibit unique characteristics that reflect their molecular structure and intermolecular interactions. For instance, the phase diagram of water (H 2O) shows a solid-liquid-gas equilibrium line that slopes upward, indicating that the melting point of ice increases with increasing pressure.
In contrast, the phase diagram of carbon dioxide (CO 2) exhibits a triple point where the solid, liquid, and gas phases coexist at a specific temperature and pressure.
Table 1 compares the phase diagrams of water, carbon dioxide, and iron:
| Compound | Triple Point (Temperature, Pressure) | Critical Point (Temperature, Pressure) |
|---|---|---|
| Water (H2O) | 0.01 °C, 0.006 atm | 374 °C, 218 atm |
| Carbon Dioxide (CO2) | -56.6 °C, 5.11 atm | 31.1 °C, 72.9 atm |
| Iron (Fe) | 1538 °C, 1 atm | – |
Factors Affecting Phase Diagrams, Puzzle ph graph of different compounds
The shape and characteristics of phase diagrams can be influenced by several factors, including:
- Molecular Structure:The molecular structure of a compound determines its intermolecular interactions, which in turn affect the phase transitions it undergoes.
- Pressure:Pressure can alter the equilibrium between phases by changing the volume of the system.
- Temperature:Temperature affects the kinetic energy of molecules, which influences their ability to overcome intermolecular forces and undergo phase transitions.
- Composition:For mixtures of compounds, the composition can affect the phase diagram by altering the intermolecular interactions between the different components.
Popular Questions: Puzzle Ph Graph Of Different Compounds
What is a phase diagram?
A phase diagram is a graphical representation of the thermodynamic conditions under which different phases of a substance can coexist in equilibrium.
How are phase diagrams constructed?
Phase diagrams are constructed experimentally by measuring the temperature, pressure, and composition of a system at which phase transitions occur.
What information can be obtained from a phase diagram?
Phase diagrams provide information about the melting point, boiling point, triple point, critical point, and other phase transitions of a substance.