Embarking on an exploration of the venn diagram of ionic and covalent bonds, this discourse delves into the captivating realm of chemical bonding, unraveling the intricate interplay between these fundamental forces that shape the molecular world.
Ionic bonds, characterized by the electrostatic attraction between oppositely charged ions, stand in contrast to covalent bonds, which arise from the sharing of electron pairs between atoms. This introductory paragraph sets the stage for a comprehensive examination of these two bond types, highlighting their unique properties and the insights they offer into the behavior of matter.
Ionic and Covalent Bonds

Ionic and covalent bonds are the two main types of chemical bonds that hold atoms together to form molecules and compounds. Ionic bonds are formed between atoms of metals and nonmetals, while covalent bonds are formed between atoms of nonmetals.Ionic
bonds are formed when one atom transfers one or more electrons to another atom. The atom that loses electrons becomes a positively charged ion, while the atom that gains electrons becomes a negatively charged ion. The oppositely charged ions are attracted to each other by the electrostatic force, forming an ionic bond.Covalent
bonds are formed when two atoms share one or more pairs of electrons. The shared electrons are attracted to the nuclei of both atoms, forming a covalent bond.
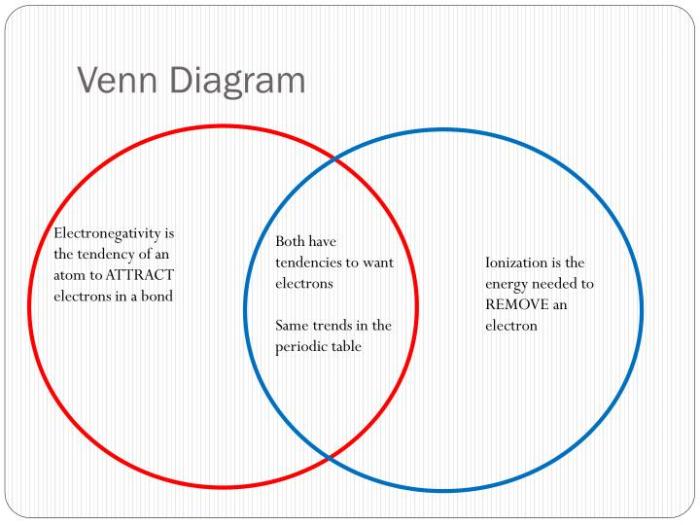
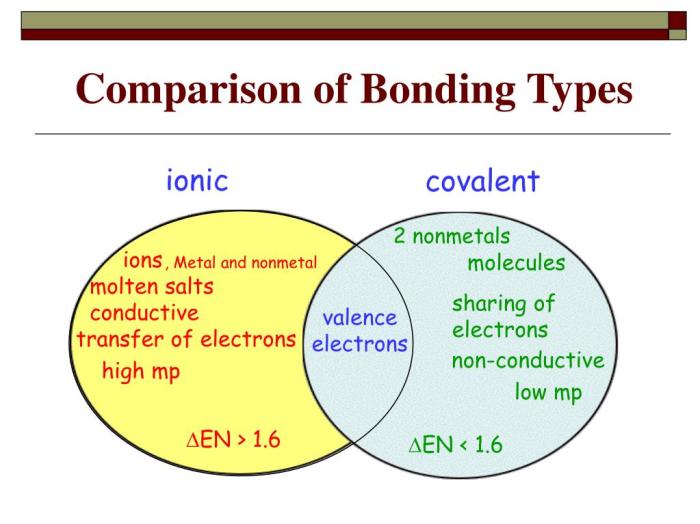
Venn Diagram
The following Venn diagram illustrates the similarities and differences between ionic and covalent bonds:Overlapping Section:
- Both ionic and covalent bonds involve the sharing of electrons between atoms.
- Both types of bonds can be strong and stable.
Non-Overlapping Sections:
-*Ionic Bonds
- Formed between metals and nonmetals.
- Involve the complete transfer of electrons from one atom to another.
- Result in the formation of charged ions.
Covalent Bonds:
- Formed between nonmetals.
- Involve the sharing of electrons between atoms.
- Do not result in the formation of charged ions.
The Venn diagram shows that ionic and covalent bonds have some similarities, but they also have some important differences.
Applications
Ionic and covalent bonds have a wide range of applications in various fields, including:
-
-*Materials Science
Ionic bonds are used in the production of ceramics, glass, and other materials. Covalent bonds are used in the production of plastics, polymers, and other materials.
-*Pharmaceuticals
Ionic bonds are used in the production of drugs and other pharmaceuticals. Covalent bonds are used in the production of vitamins and other supplements.
-*Biotechnology
Ionic bonds are used in the production of enzymes and other proteins. Covalent bonds are used in the production of DNA and other genetic material.
Polarity and Electronegativity
Polarity and electronegativity are two important factors that influence the strength and nature of ionic and covalent bonds.
-
-*Polarity
Polarity refers to the uneven distribution of electrons in a bond. In a polar bond, one atom has a greater share of the electrons than the other atom.
-*Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons. The more electronegative an atom, the more strongly it attracts electrons.
The polarity of a bond depends on the electronegativity of the atoms involved. If the atoms have the same electronegativity, the bond will be nonpolar. If the atoms have different electronegativities, the bond will be polar.Polarity and electronegativity can also affect the reactivity of molecules.
Polar molecules are more reactive than nonpolar molecules.
Bond Strength and Length, Venn diagram of ionic and covalent bonds
The strength of a bond is a measure of the energy required to break the bond. The length of a bond is the distance between the nuclei of the two atoms involved in the bond.Ionic bonds are typically stronger than covalent bonds.
This is because the electrostatic force between the oppositely charged ions is stronger than the force between the shared electrons in a covalent bond.Ionic bonds are also typically longer than covalent bonds. This is because the oppositely charged ions repel each other, causing them to be farther apart.
Molecular Geometry
The molecular geometry of a compound is determined by the number and arrangement of its atoms. Ionic and covalent bonds can affect the molecular geometry of a compound.Ionic compounds typically have a simple cubic or hexagonal crystal structure. This is because the ions are arranged in a regular pattern to maximize the electrostatic attraction between them.Covalent
compounds can have a variety of molecular geometries, depending on the number and arrangement of the atoms involved. For example, methane (CH4) has a tetrahedral molecular geometry, while water (H2O) has a bent molecular geometry.
Chemical Reactions
Ionic and covalent bonds play an important role in chemical reactions. Ionic compounds can react with each other to form new ionic compounds. Covalent compounds can react with each other to form new covalent compounds.Ionic compounds can also react with covalent compounds to form new compounds.
For example, sodium chloride (NaCl) can react with water (H2O) to form sodium hydroxide (NaOH) and hydrochloric acid (HCl).The type of chemical reaction that occurs depends on the nature of the reactants and the conditions under which the reaction is carried out.
FAQ Resource: Venn Diagram Of Ionic And Covalent Bonds
What is the key difference between ionic and covalent bonds?
Ionic bonds involve the transfer of electrons, resulting in the formation of charged ions, while covalent bonds involve the sharing of electron pairs between atoms.
How can the venn diagram of ionic and covalent bonds be used?
The venn diagram helps visualize the similarities and differences between ionic and covalent bonds, providing insights into their properties and applications.
What are some practical applications of ionic and covalent bonds?
Ionic bonds are crucial in electrolytes, batteries, and ceramics, while covalent bonds form the backbone of organic molecules and play a vital role in biological systems.