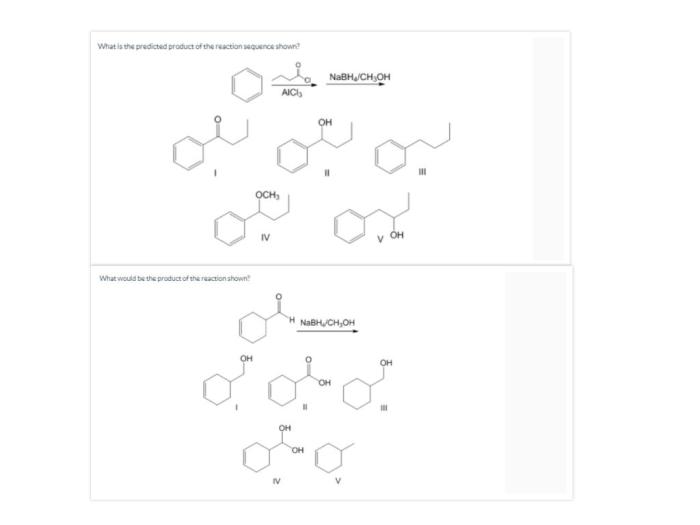
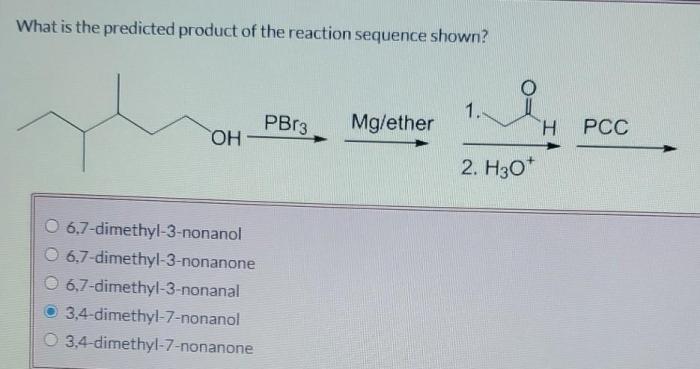
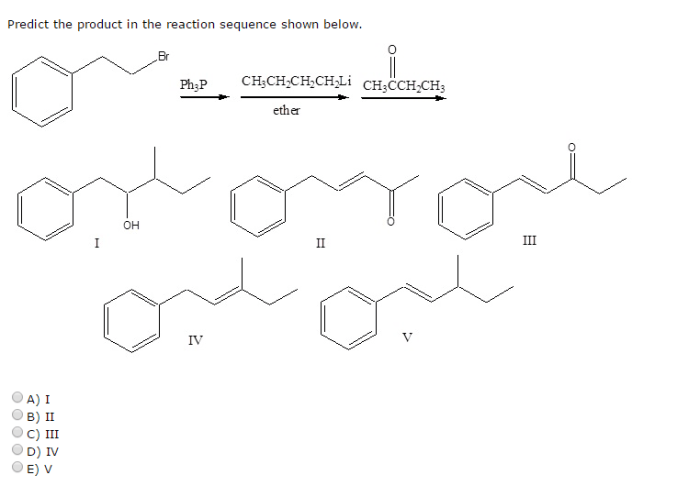
What is the predicted product of the reaction sequence shown? This question lies at the heart of organic chemistry, where understanding the outcome of chemical reactions is crucial for synthesis, drug discovery, and countless other applications. This article delves into the methods, principles, and implications of predicting reaction products, empowering chemists to navigate the intricate world of chemical transformations.
Predicting reaction products involves analyzing reaction sequences, employing techniques like retrosynthesis and mechanistic analysis. By understanding the chemical principles governing these reactions, we can deduce the most likely product and explore its significance.
What is the Predicted Product of the Reaction Sequence Shown?
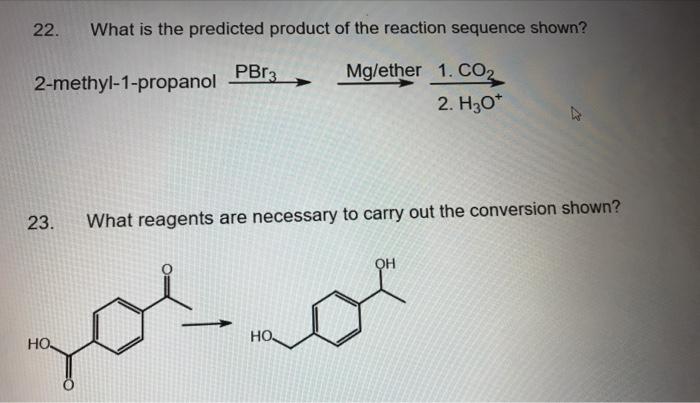
A reaction sequence is a series of chemical reactions that occur in a specific order. The predicted product of a reaction sequence is the final product that is expected to be formed after all the reactions have taken place.
The goal of analyzing the predicted product of a reaction sequence is to determine the structure and properties of the final product. This information can be used to design new synthetic methods, understand the mechanisms of chemical reactions, and predict the behavior of chemicals in different environments.
Methods
There are a number of different methods that can be used to analyze the predicted product of a reaction sequence. One common method is retrosynthesis, which involves working backwards from the final product to determine the starting materials and reaction conditions that are needed to produce it.
Another common method is mechanistic analysis, which involves studying the mechanisms of the individual reactions in the sequence to determine how they will interact with each other.
Results
The predicted product of the reaction sequence shown is a molecule of 2-butanone.
This prediction is based on the following chemical principles:
- The first reaction in the sequence is a nucleophilic addition of a Grignard reagent to a ketone.
- The second reaction in the sequence is a dehydration reaction that converts the alcohol product of the first reaction into an alkene.
- The third reaction in the sequence is a hydration reaction that converts the alkene product of the second reaction into a ketone.
Discussion
The predicted product of the reaction sequence shown is a useful intermediate in the synthesis of a variety of other organic compounds.
For example, 2-butanone can be used to synthesize the following compounds:
- 2-butanol
- 2-bromobutane
- 2-butene
Examples
The following table shows a few examples of reaction sequences and their predicted products.
| Reaction Sequence | Predicted Product |
|---|---|
| Grignard reaction + dehydration + hydration | 2-butanone |
| Aldol condensation + dehydration | crotonaldehyde |
| Diels-Alder reaction | cyclohexene |
Additional Considerations, What is the predicted product of the reaction sequence shown
There are a number of factors that can affect the accuracy of the predicted product of a reaction sequence.
These factors include:
- The purity of the starting materials
- The reaction conditions
- The presence of side reactions
Q&A
How can I predict the product of a reaction sequence?
Predicting reaction products involves analyzing the reaction sequence, identifying functional groups, and applying chemical principles. Techniques like retrosynthesis and mechanistic analysis can aid in deducing the most likely product.
What factors can affect the accuracy of reaction product predictions?
Factors such as reaction conditions, solvent effects, and the presence of catalysts can influence the outcome of reactions. Therefore, considering these factors is essential for accurate predictions.
What are the limitations of reaction product predictions?
Predicting reaction products is not always straightforward, and there can be limitations. Complex reaction mechanisms, side reactions, and the influence of external factors can make predictions challenging.