Embarking on an exploration of dry lab 3 atomic and molecular structure answers, this comprehensive guide unveils the fundamental principles governing the microscopic world. Delving into the intricate details of atomic structure, molecular bonding, and intermolecular forces, this discourse provides a profound understanding of the very building blocks of matter.
Through a meticulous examination of the fundamental particles that constitute atoms, their arrangement within atomic structures, and the energy levels and orbitals of electrons, this analysis lays the groundwork for comprehending the behavior of matter at its most basic level.
Atomic Structure
Atoms are the fundamental building blocks of matter. They are composed of three types of subatomic particles: protons, neutrons, and electrons.
Protons and neutrons are located in the nucleus of the atom, while electrons orbit the nucleus in energy levels. The number of protons in an atom determines its atomic number, which identifies the element. The number of neutrons determines the isotope of the element.
Energy Levels and Orbitals
- Electrons occupy specific energy levels around the nucleus.
- Each energy level is divided into sublevels called orbitals.
- Orbitals are three-dimensional regions where electrons are most likely to be found.
Molecular Structure
Definition of a Molecule
A molecule is a group of two or more atoms that are chemically bonded together.
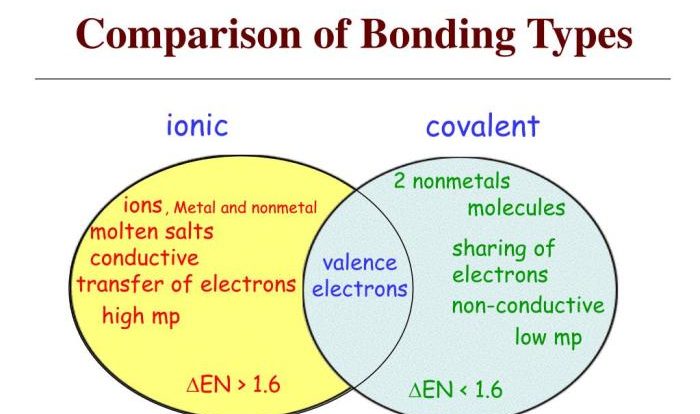
Types of Chemical Bonds
- Covalent bonds: Formed when atoms share electrons.
- Ionic bonds: Formed when one atom transfers electrons to another.
- Metallic bonds: Formed when metal atoms share their valence electrons.
Geometry and Shape of Molecules, Dry lab 3 atomic and molecular structure answers
The geometry of a molecule is determined by the arrangement of its atoms and the types of bonds between them.
The shape of a molecule can be predicted using VSEPR theory, which considers the repulsion between electron pairs.
Intermolecular Forces
Intermolecular forces are the forces that act between molecules.
The strength of these forces determines the physical properties of substances, such as their melting point, boiling point, and viscosity.
Types of Intermolecular Forces
- Van der Waals forces: Weak forces that include dipole-dipole interactions and London dispersion forces.
- Hydrogen bonding: Strong forces that occur between molecules with hydrogen atoms bonded to highly electronegative atoms.
- Dipole-dipole interactions: Forces that occur between molecules with permanent dipoles.
Applications of Atomic and Molecular Structure: Dry Lab 3 Atomic And Molecular Structure Answers

The understanding of atomic and molecular structure has led to advancements in various fields, including chemistry, biology, and materials science.
Chemistry
- Understanding chemical reactions and the properties of elements.
- Designing new materials with specific properties.
- Developing new drugs and therapies.
Biology
- Understanding the structure and function of biological molecules.
- Designing new drugs that target specific molecules.
- Developing new diagnostic tools.
Materials Science
- Understanding the properties of materials.
- Designing new materials with improved properties.
- Developing new technologies, such as solar cells and batteries.
FAQ Explained
What are the fundamental particles that make up atoms?
Atoms are composed of three fundamental particles: protons, neutrons, and electrons.
How are atoms arranged within a molecule?
Atoms are arranged within a molecule through chemical bonds, which are forces that hold atoms together.
What are the different types of chemical bonds?
There are three main types of chemical bonds: covalent bonds, ionic bonds, and metallic bonds.